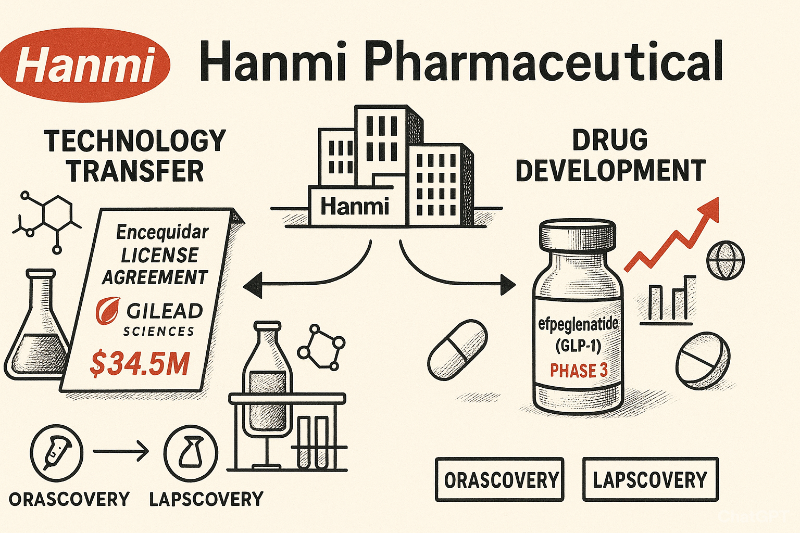
According to industry sources on the 2st, Hanmi Pharm recently signed a global technology transfer agreement with Gilead Sciences for Encequidar together with Healthhope Pharma (HHP). The total contract value is $34.5 million (approximately KRW 48.3 billion). The agreement includes a non-refundable upfront payment of $2.5 million (approximately KRW 3.5 billion) and milestones of $32 million (approximately KRW 44.8 billion).
Encequidar is an oral absorption enhancer discovered through 'Orascovery', Hanmi Pharm's proprietary drug delivery technology that converts injectable formulations to oral forms.
Through this agreement, Hanmi Pharm and HHP grant Gilead Sciences exclusive rights to Encequidar in the antiviral field. Both companies will participate as key partners in the project by supplying Encequidar active pharmaceutical ingredient (API) and finished products, and sharing technical know-how.
Hanmi Pharm previously exported 'Oraxol', an oral anticancer drug applying Encequidar, to US-based Athenex in 2011. However, following Athenex's bankruptcy, the rights were transferred to HHP. This explains why the technology transfer agreement was signed jointly with HHP.
Hanmi Pharm and HHP will receive upfront payments and milestone payments at each stage of development, approval, and sales under the agreement. They will also separately receive royalties on future product sales.
This technology transfer has increased expectations for Hanmi Pharm's performance improvement. Performance concerns have dissipated with the technology transfer agreement disclosure. Wi Hae-joo, researcher at Korea Investment & Securities, stated: "Interest in clinical momentum will increase with the resolution of performance uncertainty. Since the contract date is September 29, the upfront payment of approximately KRW 3.5 billion is expected to be recognised in the third quarter accounting."
Hanmi Pharm's first-half sales were KRW 752.2 billion, down 3.8 percent year-on-year. Operating profit also fell 11.4 percent to KRW 104.5 billion, raising performance concerns. However, this technology transfer is assessed as having laid the foundation for second-half performance recovery.
Meanwhile, New drug pipeline expansion continues. On the 30th, Hanmi Pharm submitted an Investigational New Drug (IND) application to the Ministry of Food and Drug Safety to evaluate the glycaemic control effects of combination therapy using GLP-1 obesity new drug 'efpeglenatide' with SGLT2 inhibitor and metformin (MET) in a Phase 3 clinical trial.
Efpeglenatide is a long-acting GLP-1 class treatment based on Hanmi Pharm's proprietary platform technology 'Lapscovery'. It was exported to Sanofi in 2015 for global development as a diabetes treatment. After the rights were returned in 2020, Hanmi Pharm continued independent development, rapidly expanding clinical trials as a Korean obesity new drug.
Previously, Hanmi Pharm conducted efpeglenatide clinical trials from multiple angles. The company confirmed its potential as a diabetes treatment through Phase 3 clinical trials with Sanofi targeting 6,000 type 2 diabetes patients.
The company is also developing obesity treatment 'HM17321'. This new drug substance aims to be a first-in-class obesity innovation drug that simultaneously achieves selective fat reduction and muscle mass increase.
At the recent European Association for the Study of Diabetes conference in Vienna, Austria, the company presented results that identified the molecular biological mechanism of muscle increase through muscle proteome research of animal models administered HM17321, and demonstrated glycaemic control effects through metabolic adaptation.
A Hanmi Pharm official said: "Through efpeglenatide Phase 3 clinical trials, we will provide patients with broad treatment opportunities and create achievements recognised on the global stage. We are aware of concerns about HM17321's commercialisation potential, but will continue steady development for clinical success."
Yang Hyunwoo (yhw@fntimes.com)




























![코스피 6300인데···KB금융, 12거래일 연속 외인 '순매도' 이유는 [금융지주 밸류업 점검]](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=69&h=45&m=5&simg=2026022621383900871b4a7c6999c121131189150.jpg&nmt=18)


![[속보] KAI 노조 "오늘 사장추천위 열린다"...사측 "확인 못해"](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=69&h=45&m=5&simg=20260227135534054450d260cda7511817679169.jpg&nmt=18)

!['국장 ETF' 힘으로 코스피 상승…삼성운용 질주 [ETF 통신]](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=69&h=45&m=5&simg=2026022608395305907179ad4390712813480118.jpg&nmt=18)
!['연임 특별결의' 도입 미룬 KB금융···'선제 조치' 타이틀 잡을 곳은 [2026 주총 미리보기]](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=69&h=45&m=5&simg=2026022519321609304b4a7c6999c121131189150.jpg&nmt=18)













![[그래픽 뉴스] “돈로주의 & 먼로주의: 미국 외교정책이 경제·안보에 미치는 영향”](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=298&h=298&m=1&simg=202602261105472649de68fcbb3512411124362_0.jpg&nmt=18)
![[그래픽 뉴스] 워킹맘이 바꾼 금융생활](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=298&h=298&m=1&simg=202602021638156443de68fcbb3512411124362_0.jpg&nmt=18)
![[그래픽 뉴스] 매파·비둘기부터 올빼미·오리까지, 통화정책 성향 읽는 법](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=298&h=298&m=1&simg=2026022714105702425de68fcbb3512411124362.jpg&nmt=18)
![[그래픽 뉴스] 하이퍼 인플레이션, 왜 월급이 종잇조각이 될까?](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=298&h=298&m=1&simg=202601141153149784de68fcbb3512411124362_0.jpg&nmt=18)
![[그래픽 뉴스] 주식·채권·코인까지 다 오른다, 에브리싱 랠리란 무엇일까?](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=298&h=298&m=1&simg=2026022714100604994de68fcbb3512411124362.jpg&nmt=18)
![[신간] 고수의 M&A 바이블](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=81&h=123&m=5&simg=2025091008414900330f8caa4a5ce12411124362.jpg&nmt=18)
![[신간] 리빌딩 코리아 - 피크 코리아 극복을 위한 생산성 주도 성장 전략](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=81&h=123&m=5&simg=2025032814555807705f8caa4a5ce12411124362.jpg&nmt=18)
![[서평] 추세 매매의 대가들...추세추종 투자전략의 대가 14인 인터뷰](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=81&h=123&m=5&simg=2023102410444004986c1c16452b0175114235199.jpg&nmt=18)


![[신간] 이게 화낼 일인가?](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=81&h=123&m=5&simg=2026010610254801367f8caa4a5ce12411124362.jpg&nmt=18)

![[AD] 현대차, 글로벌 안전평가 최고등급 달성 기념 EV 특별 프로모션](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=89&h=45&m=1&simg=20260106160647050337492587736121125197123.jpg&nmt=18)
![[AD] 현대차 ‘모베드’, CES 2026 로보틱스 부문 최고혁신상 수상](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=89&h=45&m=1&simg=20260105103413003717492587736121125197123.jpg&nmt=18)
![[AD] 기아 ‘PV5’, 최대 적재중량 1회 충전 693km 주행 기네스 신기록](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=89&h=45&m=1&simg=20251105115215067287492587736121125197123.jpg&nmt=18)
![[카드뉴스] KT&G, 제조 부문 명장 선발, 기술 리더 중심 본원적 경쟁력 강화](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=89&h=45&m=1&simg=202509241142445913de68fcbb3512411124362_0.png&nmt=18)
![[AD]‘황금연휴에 즐기세요’ 기아, ‘미리 추석 페스타’ 이벤트 실시](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=89&h=45&m=1&simg=20250903093618029117492587736121166140186.jpg&nmt=18)



